A team of scientists has succeeded for the first time in creating sheets of gold that are just one atom thick.
Following the naming conventions of materials science, the researchers have given this new two-dimensional material the name "goldene".
This material has some interesting properties not seen in the three-dimensional form of gold, which were presented in an article published this Thursday in the journal Nature Synthesis.
"If you make an extremely thin material, something extraordinary happens - like with graphene," explains materials scientist Shun Kashiwaya, a researcher at Linköping University in Sweden and first author of the article, in a statement published in EurekAlert.
"The same thing happens with gold. As you know, gold is normally a metal, but if it has the thickness of a layer of one atom, it can become a semiconductor," adds the scientist.
Achieving gold with a two-dimensional configuration is a major challenge because of its tendency to clump together. Previous attempts have resulted in either a thin sheet several atoms thick, or a monolayer sandwiched between or on top of another material.
Kashiwaya and his colleagues didn't set out to make goldene, but stumbled upon the first steps of their process by accident.
"We had created the basic material with completely different applications in mind," says materials physicist Lars Hultman, also a researcher at Linköping University and lead author of the study.
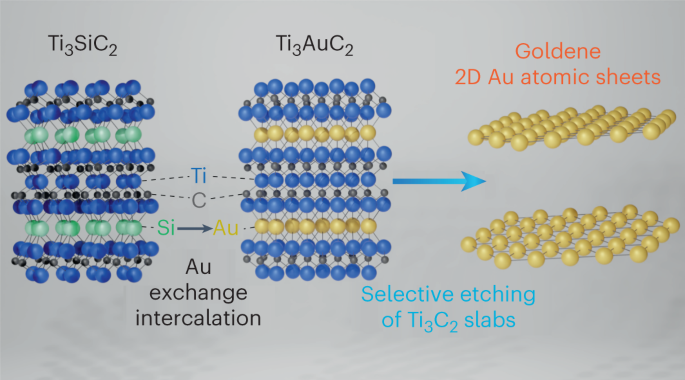
"We started with an electrically conductive ceramic called titanium silicon carbide, where the silicon is in thin layers. Then the idea was to coat the material with gold to make a contact," explains Hultman.
"But when we exposed the component to high temperatures, the silicon layer was replaced by gold inside the base material," says the researcher.
So far, so good. But, as with other attempts to create monolayer gold, progress stalled at this critical stage.
For several years, the intercalated gold-titanium carbide that the team had created was nothing more than that; there was no way of extracting the super-thin layers of gold from between the layers of titanium and carbon that sandwich it.
But in their research, the researchers experimented with applying the so-called "Murakami reagent" to a traditional "etching" technique - an engraving process in which an acid or other chemical agent is used to cut or etch lines into a surface, often used to create structures or patterns at a microscopic level on materials.
Murakami's reagent is a mixture of chemicals used in metallurgy to etch carbon and stain steel, resulting in the kind of patterns seen on some Japanese knives.
The researchers experimented with different concentrations of the mixture and different lengths of time for the corrosion process to erode the titanium and carbon around the gold. The longer they let the mixture stand, the better the results.
The corrosion effect of Murakami's reagent creates a by-product called potassium ferrocyanide. If exposed to light, the compound releases cyanide which dissolves the gold, so the engraving process had to be done entirely in the dark.
Even so, the thin sheet of gold obtained had a tendency to curl and agglomerate, which was solved by adding a surfactant that prevented the layer from folding and sticking to itself, maintaining the integrity of the monolayer.
Further analysis revealed that these complicated steps finally succeeded in forming a stable material composed of a monolayer of gold, just as the theoretical simulations had predicted.
Gold is normally an excellent conductor of electricity. When the element takes the form of a two-dimensional sheet, the atoms have two free bonds, turning it into a semiconductor with conductive properties between a conductor and an insulator, whose conductivity can be adjusted.
Gold already has properties that make it highly prized in chemical applications. Giving it the properties of a semiconductor opens up a whole new range of uses, including water purification, communication and chemical manufacturing processes.





